
Diagnosis
Renal calculus detection continues to be refined by advances in imaging technology. Helical and spiral CT scans can identify renal and ureteral calculi in an accurate and cost-effective manner. Three-dimensional reconstruction images of renal calculi is highly useful in planning PNL.
Radiolucent calculi (uric acid, crixivan, xanthine, hypoxanthine and 2,8-dihydroxyadenine calculi) are difficult to differentiate from other causes of radiolucent filling defects. Noncontrast CT scan is helpful in differentiating radiolucent stones from other pathology.
Ultrasound imaging is helpful in finding renal stones and stones at the ureterovesical junction. In the remainder of the ureter, ultrasound is poor for stone localization. Lithotriptors using ultrasound as a sole imaging device can not be used to localize ureteral stones. Intravenous contrast during ESWL (without ureteral catheterization) can also provide useful information for stone localization.
Suggested readings
Hubet J, Blum A, Cormier L, Claudon M, Regent D, Mangin P. Three-dimensional CT-scan reconstruction of renal calculi-A new tool for mapping-out staghorn calculi and follow-up of radiolucent stones. Eur Urol 1997, 31:3:297-301.
Lautin R, Gerard PS, Fuksbrumer M. Asymptomatic calculous pyelnephritits with cutaneous-renal broncho-pleural fistula. Urology 1996, 48:6:928-929.
Kim SC, Moon YT. Experience with EDAP LT02 extracorporeal shockwave lithotripsy in 1363 patients: Comparisons with results of LTOI SWL in 1586 patients. J Endourol 1997, 11:2:103-111.
Wolf JS, Bub WL, Endicott RC, Clayman RV. Use of intravenous contrast material during in situ extracorporeal shock wave lithotripsy of ureteral calculi. J Urol 1997, 157:1:38-41.
Low RK, Stoller ML. Uric acid-related nephrolithiasis. Urol Clin North Am 1997, 24:1:135-148.
Access
It is imperative to understand the pelvocaliceal anatomy before embarking on an endourological procedure. Anatomical anomalies of the urinary tract may thwart treatment efforts leading to failure and morbidity. Urolithiasis in the ectopic kidney presents special challenges to the endourologist. SWL monotherapy in the prone position results in mixed stone-free rates of 25-92%. PNL is risky due to associated anomalies in the renal vasculature. Percutaneous access to an upper calyceal stone can produce a pneumothorax, hydrothorax or hemothorax in 3% of patients. Karlin and Smith have described a technique by which a kidney is torqued in a caudal direction with a sheath in the middle pole calyx while a second sheath is introduced into an upper calyx. Suggested readings Talic RF. Extracorporeal shock-wave lithotripsy monotherapy in renal pelvic ectopia. Urol 1996, 48:6:857-861. Karlin GS, Smith AD: Approaches to the superior calyx: Renal displacement technique and review of options. J Urol 1989, 142:774-777.
Fragmentation
About 2% of renal calculi are refractory to SWL therapy. Factors influencing fragmentation include stone size, composition, radio-opacity, patient weight, and anatomy. The fragility of renal stones in increasing order is struvite, calcium apatite, uric acid, calcium oxalate dihydrate, calcium oxalate monohydrate and cystine. There is poor fragmentation of calcium oxalate monohydrate (whellelite) and cystine stones by ESWL. PNL is used to treat these difficult stones. Alternatively, the Ho:YAG laser has been successful in treating smaller renal stones via flexible ureteropyeloscopy.
Suggested readings
Lingeman JE. Extracorporeal shock wave lithotripsy: Development, instrumentation, and current status. Urol Clin North Am 1997, 24:1:185-211.
Kim HH, Lee JH, Park MS, Lee SE, Kim SW. In situ extracorporeal shockwave lithotripsy for ureteral calculi: Investigation of factors influencing stone fragmentation and appropriate number of sessions for changing treatment modality. J Endourol 1996, 10: 6: 501-505.
Clearance
The incidence of Steinstrasse secondary to SWL is low for stones less than 1cm. However, the incidence increases to 75% for large calculi. Obstruction requiring intervention occurs in 6 and 12% of cases. Although preoperative ureteral stenting is controversial, stenting may be wisely indicated in patients with solitary kidneys, unusual renal anatomy or to aid in stone visualization. In addition to stone size, stone-free rates are determined by stone location. Upper and middle calyceal calculi have a 70-90% stone-free rate, whereas, lower pole stones range between 20-70% following ESWL. Anatomical restrictions may impede the passage of stone fragments in up to 70% patients treated by ESWL. PNL is a better alternative to achieve stone-free rates in patients with ureteropelvic junction obstruction, infundibular stenosis, calyceal diverticula, ureteral obstruction, malformed kidneys (horseshoe, fused and pelvic), obstructive or large adjacent renal cysts. PNL is ideal for extraction of stones from calyceal diverticula. Alternatively, retrograde flexible ureteroscopy and laparoscopic techniques have been described for management of calyceal diverticular calculi. Suggested readings Cohen TD, Preminger GM. Management of calyceal calculi. Urol Clin North Am 1997, 24:1:81-96. Frank RG. Rupture of a large calyceal diverticulum. Urol 1997, 49:2:265-266. Chen RN, Kavoussi LR, Moore RG. Milk of calcium within a calyceal diverticulum. Urol 1997, 49:4:620-621. Wolf JS, Clayman RV. Percutaneous nephrostolithotomy: What is its role in 1997? Urol Clin North Am 1997, 24:1:43-58. Karlin GS, Smith AD: Approaches to the superior calyx: Renal displacement technique and review of options. J Urol 1989, 142:774-777.
Calyceal Diverticula
Calyceal diverticula are thin-walled cystic cavities located in the peripheral to an otherwise normal calyx, which communicate through a narrow channel. They are lined by transitional epithelium, which is the same tissue that lines the urinary tract. These cysts may originate from embryogenesis, blunt renal trauma or obstruction. They occur in any part of the kidney, but usually originate from an upper pole calyx.
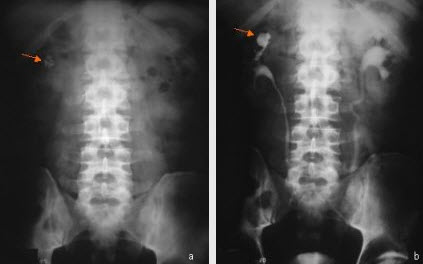
Calyceal diverticula are found in both males and females. Stones may occur in diverticulum. Thirty-six percent of diverticula were found to have stones in one series. These stones may include calcium oxalate, calcium phosphate or carbonate apatite.
Suggested readings
Cohen TD, Preminger GM. Management of calyceal calculi. Urol Clin North Am 1997, 24:1:81-96.
Abeshouse BS, Abeshouse GA: Calyceal diverticulum: A report of 16 cases and review of the literature. Urol Int 15:329, 1963.
Urinary diversion
Infection stones may occur after ureteroenteral diversion. These stones are most commonly seen between 5 and 10 years after urinary diversion surgery. The etiology of these stones may be due to infection and urea-splitting organisms, foreign body in the reservoir, mucus produced by the bowel and metabolic disturbances (hyperchloremic acidosis). The stones that form may be composed of a mixture of magnesium ammonium phosphate, carbonate apatite, calcium phosphate and calcium oxalate.
Augmentation cystoplasty
Cystoplasty For patients with small contracted bladders, bowel segments can be used to increase the bladder capacity for storage purposes. One of the major complications of bladder augmentation is the formation of bladder calculi. The incidence of calculus formation in augmentative bladders is between 18-50%. Possible metabolic causes implicated in the formation of stones in these patients include intestinal mucus as a lithogenic agent and altered levels of inhibitors. Citrate, a known inhibitor of crystallization, works by its ability to chellate calcium, thereby reducing calcium salt formation. Therefore, reduced citrate levels in the urine are implicated in bladder stone formation.
Bladder Stones
A bladder stone, also referred to as vesical calculus, is predominantly a disease of men. In the United States, bladder stones occur usually in men over the age of 50 and are usually associated with bladder outlet obstruction. The diagnosis of a bladder stone should result in a complete urologic evaluation for factors that result in retention of urine, including stricture of the urethra, benign prostatic hyperplasia, diverticulum of the bladder, and neurogenic bladder.
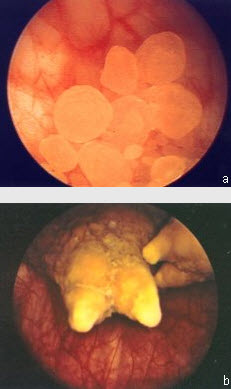
In contrast to renal stones, bladder stones are usually composed of uric acid stones (see Figure a) in noninfected urine or struvite stones (See Figure b) in infected urine.
Symptoms
In patients with prostatic enlargement and residual urine, the complaints may be those of prostatic obstruction, with the calculus being found incidentally. Typical symptoms of bladder stone are intermittent, painful voiding with terminal hematuria (blood at the end of the urinary stream). Discomfort may be dull, aching, or sharp suprapubic pain, which is aggravated by exercise and sudden movement. Severe pain usually occurs near the end of micturition, as the stone impacts at the bladder neck. Relief may be afforded by assuming a recumbent position. The pain may be referred to the tip of the penis, scrotum, or the perineum and on occasion to the back, the hip or even the heel or sole of the foot. Besides pain, there may be an interruption of the urinary stream from impaction of the stone at the bladder neck or urethra. Priapism and nocturnal enuresis may occur in children.
Diagnosis
Bladder stones are not often seen on plain films because of the presence of uric acid in many of the calculi and because of overlying prostatic tissue. However, struvite stones, which are radiopaque, may be seen on plain film (See Figure c). Ultrasonography is useful for detecting radiolucent calculi. Cystoscopic examination is the best method for detecting bladder stones.
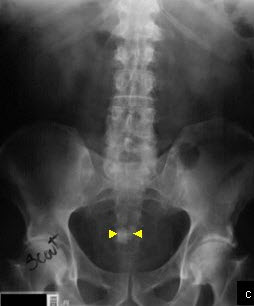
Bladder calculus. Plain abdominal film demonstrates densely opaque, calculus in the pelvis (yellow arrows). Cystoscopy confirmed the presence of a bladder stone.
Renal calculus detection continues to be refined by advances in imaging technology. Helical and spiral CT scans can identify renal and ureteral calculi in an accurate and cost-effective manner. Three-dimensional reconstruction images of renal calculi is highly useful in planning PNL.
Radiolucent calculi (uric acid, crixivan, xanthine, hypoxanthine and 2,8-dihydroxyadenine calculi) are difficult to differentiate from other causes of radiolucent filling defects. Noncontrast CT scan is helpful in differentiating radiolucent stones from other pathology.
Ultrasound imaging is helpful in finding renal stones and stones at the ureterovesical junction. In the remainder of the ureter, ultrasound is poor for stone localization. Lithotriptors using ultrasound as a sole imaging device can not be used to localize ureteral stones. Intravenous contrast during ESWL (without ureteral catheterization) can also provide useful information for stone localization.
Suggested readings
Hubet J, Blum A, Cormier L, Claudon M, Regent D, Mangin P. Three-dimensional CT-scan reconstruction of renal calculi-A new tool for mapping-out staghorn calculi and follow-up of radiolucent stones. Eur Urol 1997, 31:3:297-301.
Lautin R, Gerard PS, Fuksbrumer M. Asymptomatic calculous pyelnephritits with cutaneous-renal broncho-pleural fistula. Urology 1996, 48:6:928-929.
Kim SC, Moon YT. Experience with EDAP LT02 extracorporeal shockwave lithotripsy in 1363 patients: Comparisons with results of LTOI SWL in 1586 patients. J Endourol 1997, 11:2:103-111.
Wolf JS, Bub WL, Endicott RC, Clayman RV. Use of intravenous contrast material during in situ extracorporeal shock wave lithotripsy of ureteral calculi. J Urol 1997, 157:1:38-41.
Low RK, Stoller ML. Uric acid-related nephrolithiasis. Urol Clin North Am 1997, 24:1:135-148.
It is imperative to understand the pelvocaliceal anatomy before embarking on an endourological procedure. Anatomical anomalies of the urinary tract may thwart treatment efforts leading to failure and morbidity. Urolithiasis in the ectopic kidney presents special challenges to the endourologist. SWL monotherapy in the prone position results in mixed stone-free rates of 25-92%. PNL is risky due to associated anomalies in the renal vasculature. Percutaneous access to an upper calyceal stone can produce a pneumothorax, hydrothorax or hemothorax in 3% of patients. Karlin and Smith have described a technique by which a kidney is torqued in a caudal direction with a sheath in the middle pole calyx while a second sheath is introduced into an upper calyx. Suggested readings Talic RF. Extracorporeal shock-wave lithotripsy monotherapy in renal pelvic ectopia. Urol 1996, 48:6:857-861. Karlin GS, Smith AD: Approaches to the superior calyx: Renal displacement technique and review of options. J Urol 1989, 142:774-777.
About 2% of renal calculi are refractory to SWL therapy. Factors influencing fragmentation include stone size, composition, radio-opacity, patient weight, and anatomy. The fragility of renal stones in increasing order is struvite, calcium apatite, uric acid, calcium oxalate dihydrate, calcium oxalate monohydrate and cystine. There is poor fragmentation of calcium oxalate monohydrate (whellelite) and cystine stones by ESWL. PNL is used to treat these difficult stones. Alternatively, the Ho:YAG laser has been successful in treating smaller renal stones via flexible ureteropyeloscopy.
Suggested readings
Lingeman JE. Extracorporeal shock wave lithotripsy: Development, instrumentation, and current status. Urol Clin North Am 1997, 24:1:185-211.
Kim HH, Lee JH, Park MS, Lee SE, Kim SW. In situ extracorporeal shockwave lithotripsy for ureteral calculi: Investigation of factors influencing stone fragmentation and appropriate number of sessions for changing treatment modality. J Endourol 1996, 10: 6: 501-505.
The incidence of Steinstrasse secondary to SWL is low for stones less than 1cm. However, the incidence increases to 75% for large calculi. Obstruction requiring intervention occurs in 6 and 12% of cases. Although preoperative ureteral stenting is controversial, stenting may be wisely indicated in patients with solitary kidneys, unusual renal anatomy or to aid in stone visualization. In addition to stone size, stone-free rates are determined by stone location. Upper and middle calyceal calculi have a 70-90% stone-free rate, whereas, lower pole stones range between 20-70% following ESWL. Anatomical restrictions may impede the passage of stone fragments in up to 70% patients treated by ESWL. PNL is a better alternative to achieve stone-free rates in patients with ureteropelvic junction obstruction, infundibular stenosis, calyceal diverticula, ureteral obstruction, malformed kidneys (horseshoe, fused and pelvic), obstructive or large adjacent renal cysts. PNL is ideal for extraction of stones from calyceal diverticula. Alternatively, retrograde flexible ureteroscopy and laparoscopic techniques have been described for management of calyceal diverticular calculi. Suggested readings Cohen TD, Preminger GM. Management of calyceal calculi. Urol Clin North Am 1997, 24:1:81-96. Frank RG. Rupture of a large calyceal diverticulum. Urol 1997, 49:2:265-266. Chen RN, Kavoussi LR, Moore RG. Milk of calcium within a calyceal diverticulum. Urol 1997, 49:4:620-621. Wolf JS, Clayman RV. Percutaneous nephrostolithotomy: What is its role in 1997? Urol Clin North Am 1997, 24:1:43-58. Karlin GS, Smith AD: Approaches to the superior calyx: Renal displacement technique and review of options. J Urol 1989, 142:774-777.
Calyceal diverticula are thin-walled cystic cavities located in the peripheral to an otherwise normal calyx, which communicate through a narrow channel. They are lined by transitional epithelium, which is the same tissue that lines the urinary tract. These cysts may originate from embryogenesis, blunt renal trauma or obstruction. They occur in any part of the kidney, but usually originate from an upper pole calyx.

Calyceal diverticula are found in both males and females. Stones may occur in diverticulum. Thirty-six percent of diverticula were found to have stones in one series. These stones may include calcium oxalate, calcium phosphate or carbonate apatite.
Suggested readings
Cohen TD, Preminger GM. Management of calyceal calculi. Urol Clin North Am 1997, 24:1:81-96.
Abeshouse BS, Abeshouse GA: Calyceal diverticulum: A report of 16 cases and review of the literature. Urol Int 15:329, 1963.
Infection stones may occur after ureteroenteral diversion. These stones are most commonly seen between 5 and 10 years after urinary diversion surgery. The etiology of these stones may be due to infection and urea-splitting organisms, foreign body in the reservoir, mucus produced by the bowel and metabolic disturbances (hyperchloremic acidosis). The stones that form may be composed of a mixture of magnesium ammonium phosphate, carbonate apatite, calcium phosphate and calcium oxalate.
Cystoplasty For patients with small contracted bladders, bowel segments can be used to increase the bladder capacity for storage purposes. One of the major complications of bladder augmentation is the formation of bladder calculi. The incidence of calculus formation in augmentative bladders is between 18-50%. Possible metabolic causes implicated in the formation of stones in these patients include intestinal mucus as a lithogenic agent and altered levels of inhibitors. Citrate, a known inhibitor of crystallization, works by its ability to chellate calcium, thereby reducing calcium salt formation. Therefore, reduced citrate levels in the urine are implicated in bladder stone formation.
A bladder stone, also referred to as vesical calculus, is predominantly a disease of men. In the United States, bladder stones occur usually in men over the age of 50 and are usually associated with bladder outlet obstruction. The diagnosis of a bladder stone should result in a complete urologic evaluation for factors that result in retention of urine, including stricture of the urethra, benign prostatic hyperplasia, diverticulum of the bladder, and neurogenic bladder.
In contrast to renal stones, bladder stones are usually composed of uric acid stones (see Figure a) in noninfected urine or struvite stones (See Figure b) in infected urine.

Symptoms
In patients with prostatic enlargement and residual urine, the complaints may be those of prostatic obstruction, with the calculus being found incidentally. Typical symptoms of bladder stone are intermittent, painful voiding with terminal hematuria (blood at the end of the urinary stream). Discomfort may be dull, aching, or sharp suprapubic pain, which is aggravated by exercise and sudden movement. Severe pain usually occurs near the end of micturition, as the stone impacts at the bladder neck. Relief may be afforded by assuming a recumbent position. The pain may be referred to the tip of the penis, scrotum, or the perineum and on occasion to the back, the hip or even the heel or sole of the foot. Besides pain, there may be an interruption of the urinary stream from impaction of the stone at the bladder neck or urethra. Priapism and nocturnal enuresis may occur in children.
Diagnosis
Bladder stones are not often seen on plain films because of the presence of uric acid in many of the calculi and because of overlying prostatic tissue. However, struvite stones, which are radiopaque, may be seen on plain film (See Figure c). Ultrasonography is useful for detecting radiolucent calculi. Cystoscopic examination is the best method for detecting bladder stones.

Bladder calculus. Plain abdominal film demonstrates densely opaque, calculus in the pelvis (yellow arrows). Cystoscopy confirmed the presence of a bladder stone.
